At the end of 2021, Glory Fund completed an investment of tens of millions of RMB in BeBetter Med Inc. (hereinafter “BeBetter Med”). Proceeds from this financing round will be primarily used for clinical research and the commercialization of BeBetter Med’s new-drug pipeline.

Glory Fund’s comments
Glory Fund believes: “Against a policy backdrop constrained by centralized procurement and similar measures, China’s biopharmaceutical industry is increasingly focusing on the innovation track. That Bebetter Med has emerged from a large pool of oncology R&D companies is itself a recognition of the strength of its pipeline and core team. Facing China — the world’s second-largest pharmaceutical market — and the vast opportunities for domestic substitution of innovative drugs, the future of China’s innovative biopharma companies is set to take off. We are optimistic about several of Bebetter Med’s pipelines, especially the First-in-Class candidate BEBT-908 and its future development. The global market holds enormous potential, and Glory Fund is willing to share the growth dividends together with many China-based innovative pharmaceutical and technology enterprises.”
BeBetter Med Inc. was founded in 2012 and is primarily engaged in innovative drug research, development and technology transfer. Its objective is to develop advanced drugs to treat cancer and other serious diseases. The company has capabilities spanning chemical synthesis and new-drug discovery through preclinical research to clinical development. It also possesses core technologies for proprietary drug design and development. Since its establishment, the company has been granted 13 Chinese invention patents and has filed or published 10 international invention patent applications. To date, Bebetter Med has built a pipeline containing 11 Class-I new drugs, including five clinical-stage candidates,
BEBT-908 — a globally pioneering Class-I new drug that is a PI3K/HDAC dual-target inhibitor (currently in a Phase II clinical trial for lymphoma). It has demonstrated significant antitumor efficacy and favorable safety.
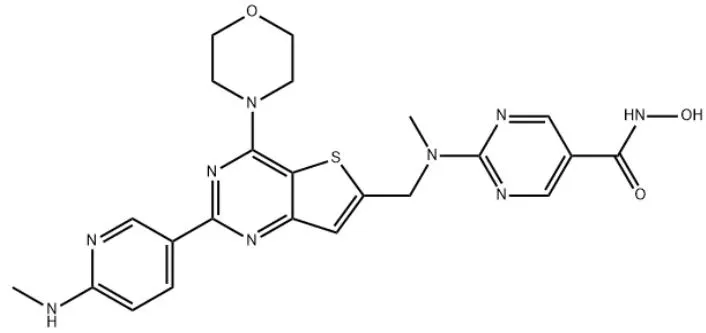
Chemical formula of BEBT-908
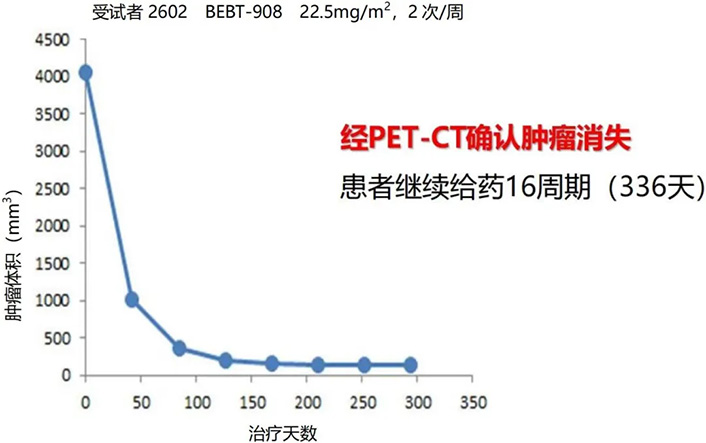
Favorable pharmacodynamic results of BEBT-908 in a Phase I clinical trial for hematologic and lymphoid malignancies.
BEBT-109 — the world’s first pan-EGFR mutant kinase inhibitor, with high inhibitory activity against common EGFR mutations and various rare mutations; it may achieve breakthroughs on the crowded EGFR track by targeting exon 20 insertion mutations and other rare variants.
BEBT-305 — the world’s first chaperone inhibitor for the treatment of psoriasis. European partner-conducted Ib trial results showed it to be safe and effective in moderate-to-severe psoriasis.
BEBT-260 — the first Chk1 cell-cycle checkpoint inhibitor in China to enter clinical development. It shows clear advantages in compound pharmacokinetics and safety compared with similar drugs at foreign Ib/II stages and may become the world’s first approved drug of its kind.
BEBT-503 — to date the most potent pan-PPAR agonist, with potential to achieve a major breakthrough in treating diabetes with coexisting NASH (nonalcoholic steatohepatitis).
Focusing on oncology, autoimmune, metabolic, and other major diseases, Bebetter Med has independently developed several Class-I original new drugs, aiming for First-in-Class and Best-in-Class status to address significant unmet clinical needs and benefit millions of patients. After years of hard work, the company has become one of China’s leading innovative drug enterprises and is expected to bring multiple Class-I new drugs to market within the next 5–10 years.
Currently, Bebetter Med’s clinical-stage programs are all Class-I innovative oncology drugs targeting relapsed/refractory or otherwise untreatable advanced/metastatic malignancies, with the potential for conditional approval following successful pivotal Phase II trials. Programs at the IND-filing and preclinical stages are focused on cutting-edge oncology targets and major disease markets, advancing in parallel with a long-term view. Core patents for all projects in clinical and IND stages have been granted or filed; some have been or are being authorized in multiple countries and regions, including Europe, the U.S., and Japan.
On January 24, 2022, Bebetter Med formally submitted IPO counseling/filing materials to the Guangdong Regulatory Bureau of the China Securities Regulatory Commission. On January 25, 2022, the Guangdong Regulatory Bureau accepted the filing.